Adsorption
Adsorption is a mass transfer process in which a substance is transported from the liquid phase to the surface of a solid/liquid (adsorbent) and becomes physically and chemically bonded (adsorbate). Adsorption can be classified into two forms based on the type of attraction between the adsorbate and the adsorbent: physical and chemical adsorption, commonly known as physisorption and chemisorption.
Activated Carbon
Activated carbons are effective adsorbents for a wide variety of contaminants. The adsorptive removal of color, aroma, taste, and other harmful organics and inorganics from drinking water and wastewater is one of their industrial applications.
Both a high surface area and a large pore size can improve the efficiency of activated carbon. Activated carbon was utilized by a number of studies to remove heavy metals and other types of contaminants from wastewater.
Biological Treatment
This is the method by which dissolved and suspended organic chemical components are eliminated through biodegradation, in which an optimal amount of microorganism is given to re-enact the same natural self-purification process.
Through two distinct biological process, such as biological oxidation and biosynthesis, microorganisms can degrade organic materials in wastewater. Microorganisms involved in wastewater treatment produce end products such as minerals, carbon dioxide, and ammonia during the biological oxidation process. The minerals (products) remained in the wastewater and were discharged with the effluent. Microorganisms use organic materials in wastewater to generate new microbial cells with dense biomass that is eliminated by sedimentation throughout the biosynthesis process.
Bioremediation
Bioremediation is a biological treatment method in which micro-organisms breakdown or transform hazardous contaminants in wastewater to a less toxic or non-toxic state. The following technologies can be used to bioremediate wastewater, which can be done by autotrophs or heterotrophs .Slow sand filtration using a biofilm to metabolize organic matter, adsorb soluble components and entrap particulates.
Technologies
Technologies for potable water and other uses are well-developed, and generalized designs are available from which treatment processes can be selected for pilot testing on the specific source water. In addition, a number of private companies provide patented technological solutions for the treatment of specific contaminants. Automation of water treatment is common in the developed world. Source water quality through the seasons, scale, and environmental impact can dictate capital costs and operating costs. End use of the treated water dictates the necessary quality monitoring technologies, and locally available skills typically dictate the level of automation adopted.
Desalination
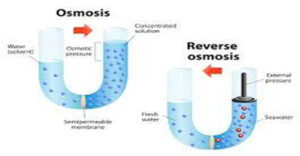
Saline water can be treated to yield fresh water. Two main processes are used, reverse osmosis or distillation. Both methods require more energy than water treatment of local surface waters and are usually only used in coastal areas or where water such as groundwater has high salinity.
Portable water purification
Living away from drinking water supplies often requires some form of portable water treatment process. These can vary in complexity from the simple addition of a disinfectant tablet in a hiker's water bottle through to complex multi-stage processes carried by boat or plane to disaster areas.
| Constituent |
Unit processes |
| Turbidity and particles |
Coagulation/ flocculation, sedimentation, granular filtration |
| Major dissolved inorganics |
Softening, aeration, membranes |
| Minor dissolved inorganics |
Membranes |
| Pathogens |
Sedimentation, filtration, disinfection |
| Major dissolved organics |
Membranes, adsorption |
Standards
Main article: Drinking water quality standards
enforcement.] Two exceptions are the European Drinking Water Directive and the Safe Drinking Water Act in the United States, which require legal compliance with specific standards.
Industrial water treatment
Processes
Two of the main processes of industrial water treatment are: -
boiler water treatment and cooling water treatment. A large amount of proper water treatment can lead to the reaction of solids and bacteria within pipe work and boiler housing. Steam boilers can suffer from scale or corrosion when left untreated.
Scale deposits can lead to weak and dangerous machinery, while additional fuel is required to heat the same level of water because of the rise in thermal resistance. Poor quality dirty water can become a breeding ground for bacteria such as Legionella causing a risk to public health.
Corrosion in low pressure boilers can be caused by dissolved oxygen, acidity and excessive alkalinity. Water treatment therefore should remove the dissolved oxygen and maintain the boiler water with the appropriate pH and alkalinity levels. Without effective water treatment, a cooling water system can suffer from scale formation, corrosion and fouling and may become a breeding ground for harmful bacteria. This reduces efficiency, shortens plant life and makes operations unreliable and unsafe.
Boiler water treatment
Boiler water treatment is a type of industrial water treatment focused on removal or chemical modification of substances potentially damaging to the boiler. Varying types of treatment are used at different locations to avoid scale, corrosion, or foaming. External treatment of raw water supplies intended for use within a boiler is focused on removal of impurities before they reach the boiler. Internal treatment within the boiler is focused on limiting the tendency of water to dissolve the boiler and maintaining impurities in forms least likely to cause trouble before they can be removed from the boiler in boiler blowdown.
Cooling water treatment
Water cooling is a method of heat removal from components of machinery and industrial equipment. Water may be a more efficient heat transfer fluid where air cooling is ineffective. . Chemical additives to reduce these disadvantages may introduce toxicity to wastewater. Water cooling is commonly used for cooling automobile internal combustion engines and large industrial facilities such as nuclear and steam electric power plants, hydroelectric generators, petroleum refineries and chemical plants.
Technologies
Chemical treatment
Chemical treatments utilizes the additive of chemicals to make industrial water suitable for use or discharge. These includes processes like-
- chemical precipitation,
- chemical disinfection,
- Advanced oxidation process (AOP),
- ion exchange,
- and chemical neutralization
Physical treatment
Physical treatment involves the separation of solids form industrial wastewater either through Filtration or Dissolved air flotation. Filtration involves the use of Membrane or filters such as mechanical filters like sand filtration etc to achieve solid-liquid separation. Whereas for Dissolved air flotation,
pressurized air is pumped into the wastewater. The pressurized air then forms small bubbles which adhere to the suspended matter causing them to float to the surface of the water where they can be removed by a skimming device or an overflow.
Biological treatment
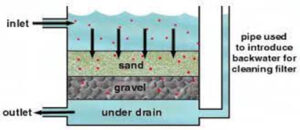
Slow sand filters use a biological process to purify raw water to produce potable water. They work by using a complex biological film that grows naturally on the surface of sand. This gelatinous biofilm called the hypogeal layer is located in the upper few millimetres of the sand layer.
The surface biofilm purifies the water as it flows through the layer, the underlying sand provides a support medium for the biological treatment layer. The Schmutzdecke consists of bacteria, fungi, protozoa, rotifera and a range of aquatic insect larvae. As the biofilm ages, more algae may develop and larger aquatic organisms including bryozoa, snails and Annelid worms may be present. As water passes through the hypogeal layer, particles of matter are trapped in the mucilaginous matrix and soluble organic material is adsorbed. The contaminants are metabolised by the bacteria, fungi and protozoa.
Slow sand filters are typically 1–2 metres deep and have a hydraulic loading rate of 0.2–0.4 cubic metres per square metre per hour. Filters lose their performance as the biofilm thickens and reduces the rate of flow. The filter is refurbished by removing the biofilm and a thin upper layer of sand. Water is decanted back into the filter and re-circulated to enable a new biofilm to develop. Alternatively wet harrowing involves stirring the sand and flushing the biolayer through for disposal.
Physio-chemical treatment: -
Chemical flocculants are used to generate a floc in the water that traps suspended solids. Chemical polyelectrolytes are used to increase coagulation of suspended solids to improve removal.
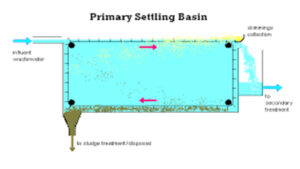
This consist of a primary coagulant such as ferric sulphate and a coagulant aid cationic polymer being flash-mixed before it enters a Flocculation Basin.
Once the source water being treated has been flash-mixed with a primary coagulant and a polymer, they are then put into some type of flocculation basin, where slow turning or mixing of the water, mixes the chemicals together and they can then form what is called "floc" .
- After the water has mixed and the floc has formed, it is then passed to the next stage which would be the settling The water would flow up through these tubes or plates, allowing the clear water to flow over into an effluentlaunder, which would then carry the "settled" water to the filters for further treatment.
- The tubes/plates in the settling stage, allow a greater surface area for the "floc" to settle on. These plates are typically at a 30–45° angle, allowing the floc particles to collect in the tubes or the plates and eventually ending up in the bottom of the settling basin.
- There is typically some sort of sludgecollection system that then will collect all of the settled floc a.k.a. sludge, and pump it or transfer the waste to a decant tank or basin, where it is later disposed of.
- Once the settled water had travelled to the filters, and has made its way through the filters, it is then stored in a clear well, where all the filtered water gets collected for additional chemical addition: pHadjuster, chlorine or UV light.
- After the appropriate contact timeor kill time, the water leaves the clear well and heads out to storage tanks or into the distribution, all the way to the customers faucet for us.
Uses of recycled or treated water: -
- after treatment the treated water may be released to a sanitary surface water in the environment.
- We can use it in the agriculture, landscape irrigation, industrial processes.
- Non portable water uses like fire protection ground water recharge, recreation, gardening, cleaning, washing purposes
- Electricity production.








Leave a Reply